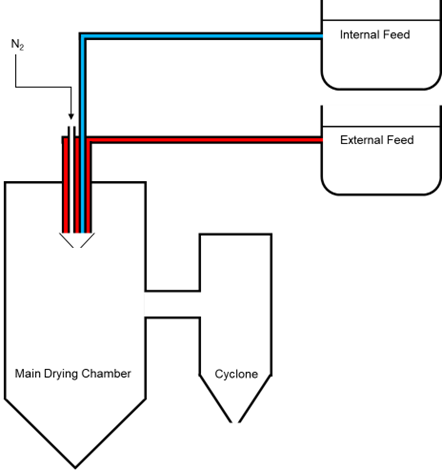
The spray drying facility includes state-of-the-art formulation technologies such as:
- Novel spray drying technologies to prepare drugs to improve physicochemical properties. The process can be particularly helpful for scaling up studies, to improve solubility, to prepare amorphous drugs and to prepare biological formulations. We host latest state-of-the-art technology using two and three fluid nozzle systems allowing spray drying aqueous and organic based solutions.
- A range of mechanical activation equipment to do milling to prepare particles with desired particle size range.
- Tableting to prepare tablets with optimum dissolution and release properties.
The spray drying facility can conduct formulation and pre-formulation studies of oral and inhaled products. We host in vitro facilities to assess flow properties and dissolution of oral and oral inhaled drugs. This includes:
- In-situ particle size measurement using Focused Beam Reflectance Measurement (FBRM)
- Andersen Cascade Impactor (ACI)
- USP 2 and 4 equipment to test dissolution
- HPLC and UV based methods
- Rheology of fluids
We host a range of facilities within CAF with broad applications for physiochemical analysis. The facilities include: Thermal, optical, gravimetric and XRPD (full details can be found on our facilities and equipment page).
