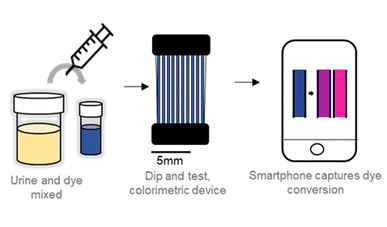
Inflammation-based point-of-care (PoC) diagnostic innovations are critical to prevent unnecessary antibiotic use and the development of antimicrobial resistance. Our research team confirmed that miniaturised phenotypic antibiotic susceptibility tests (AST) are compatible with conventional methods. Direct testing (without isolation) is particularly feasible for urinary tract infections, enabling the use of microfluidic AST systems in PoC. As the bacterial growth rate is temperature dependent, temperature control and mass production of microfluidic test strips in PoC are required.
This study demonstrates the application of direct microcapillary AST (mcAST) from clinical specimens with minimal equipment and a smartphone camera. The test on 12 clinical specimens achieved 100% accuracy in detecting bacteria in urine and 95% categorical agreement with 4 antibiotics. The kinetic model showed that the degradation time of resazurin in microcapillaries was dependent on bacterial density. Furthermore, mass production of mcAST strips by air drying gave results in agreement with standard AST methods. These results bring mcAST closer to clinical application and make it possible to support antibiotic prescription decisions within one day at PoC.
Check out the web link below to access the paper!