Microfluidic Antimicrobial Resistance Tests
At EdwardsLab we use microfluidic devices to develop rapid antimicrobial resistance
tests for UTI infections. These tests use a hydrophillic microcapillary film with
different capillaries coated with antibiotics. The urine sample is then mixed with a
metabolic dye that turns from blue to pink
or from non-fluorescent to fluroescent in
the presence of bacteria.
Microcapillary film
Using these hydrophillic microcapillary film strips, a single well of a 96 well plate can be expanded to 10 individual tests. We use 3D printing rapid prototyping to make accessories for the microcapillary strips like the clip on ladder.


Time-lapse imaging with low-cost hardware
Using timelapse imaging and microcapillaries allows us to
- Determine the starting concentration of bacteria in an unknown sample
- Rapidly determine susceptability profiles for different antibiotics
To monitor our experiments we use low-cost hardware such as raspberry pi computers and cameras that we can program to take images at specific time points. We have added a raspberry pi camera to a 3D printer frame. This allows us to switch on LEDs, take a picture and move over an imaging area to increase out throughput of experiments.
Take a look at our PLOS One publication on low-cost raspberry pi based imaging setup here.
Home usability of diagnostic devices
Why use home testing devices?
When diagnosing a virus or a bacterial infection, or even finding out what type of antibiotic would be suitable, home testing could provide a quick way to get results to as many people as possible.
Even in situations where home testing is not necessary, rapid point-of-care diagnoses can still be vital to deliver fast medical action, e.g. tackling widespread health crises in rural areas with low wealth. Sometimes these tests are carried out by healthcare professionals, but often users have little or no training on how to use the devices, and it is important that users can follow instructions and interpret tests accurately to recommend an appropriate medical outcome.
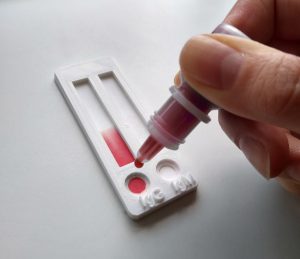
What is usability?
Whether it is the ability of the user to understand and follow the written instructions, or interpret the result of the test, the usability is a measure of how accurately the device is used.
Studying usability can:
- Guide device design, including what tools are used to collect and transfer the sample
- Improve instructions given, whether those be exclusively written instructions, using images to aid instuctions, or an instructional video
- Simply give an insight into how the accuracy of the test will vary depending on the user error

Why study usability remotely?
The original intent of this study was to study point-of-care usability of lateral flow devices, similar to those used to diagnose Dengue (and not too dissimilar to the pregnancy test that many will be familiar with the concept of). However, after lockdown measures were imposed during the COVID-19 outbreak, there were suggestions of home testing for COVID-19 using lateral flow devices that were only previously used by trained personnel, and would need to be approved before use. The ability for researchers to study usability of diagnostic devices remotely became a clear area of interest.
Most research studies involve many volunteers coming to a central location to test devices, which would pose an increased risk of infection for those taking part. By studying usability remotely, the risk of infection can be reduced, and usability can be carried out during any level of lockdown status.
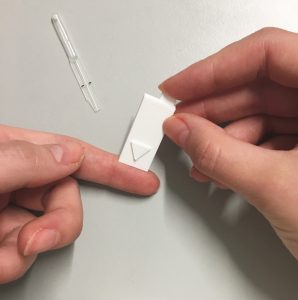
What are we doing?
Our goal is to find out how feasible it would be to study usability remotely, using video calls and mock diagnostic devices. We have 3D printed mock lancets (for taking blood), and lateral flow devices, and delivered these to volunteers houses alongside a bottle of dye (fake blood) and sampling pipettes to measure accuracy of sample volume transfer. We can remotely assess how well users can follow simple written instructions when using a dual lateral flow device, how accurate various steps are followed, and how we can quantify volume of dye administered using images taken on smartphone devices.
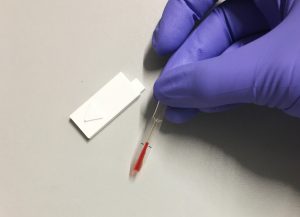
Even outside of the context of the coronavirus crisis, this would still be a useful capability for assessing usability studies in remote locations, and for including volunteers who may have been previously excluded from studies due to ability to travel to the testing location.
