EPR OTTLE Cell
Published here in (Collect. Czech. Chem. Commun., 2001, 66, 52-66) and based off of work by Allendoerfer (1975) our electron paramagnetic resonance cell offers unparalleled performance, allowing users to both generate and stabilize the sometimes highly sensitive paramagnetic species.
Electron Paramagnetic Resonance OTTLE Cell
To support our users in their investigations of paramagnetic species our cell has a number of impressive features.
- Coaxial design allows for a Au helical working & Pt helical counter electrode to be placed into the EPR cavity.

- Boasts a high surface area for rapid electrolysis and undistorted EPR signal.
- The potential at the WE is easily controlled by a Ag psuedo-reference electrode, placed just above the working electrode.
- Placement of the electrodes in the EPR cavity allows the cell to be evacuated – preventing decomposition of the radical species through exposure to moisture and air.
- Moreover, the cell can be simply cooled by placing into a Dewar-type holder which is preventing decomposition of the radical species through thermal means.
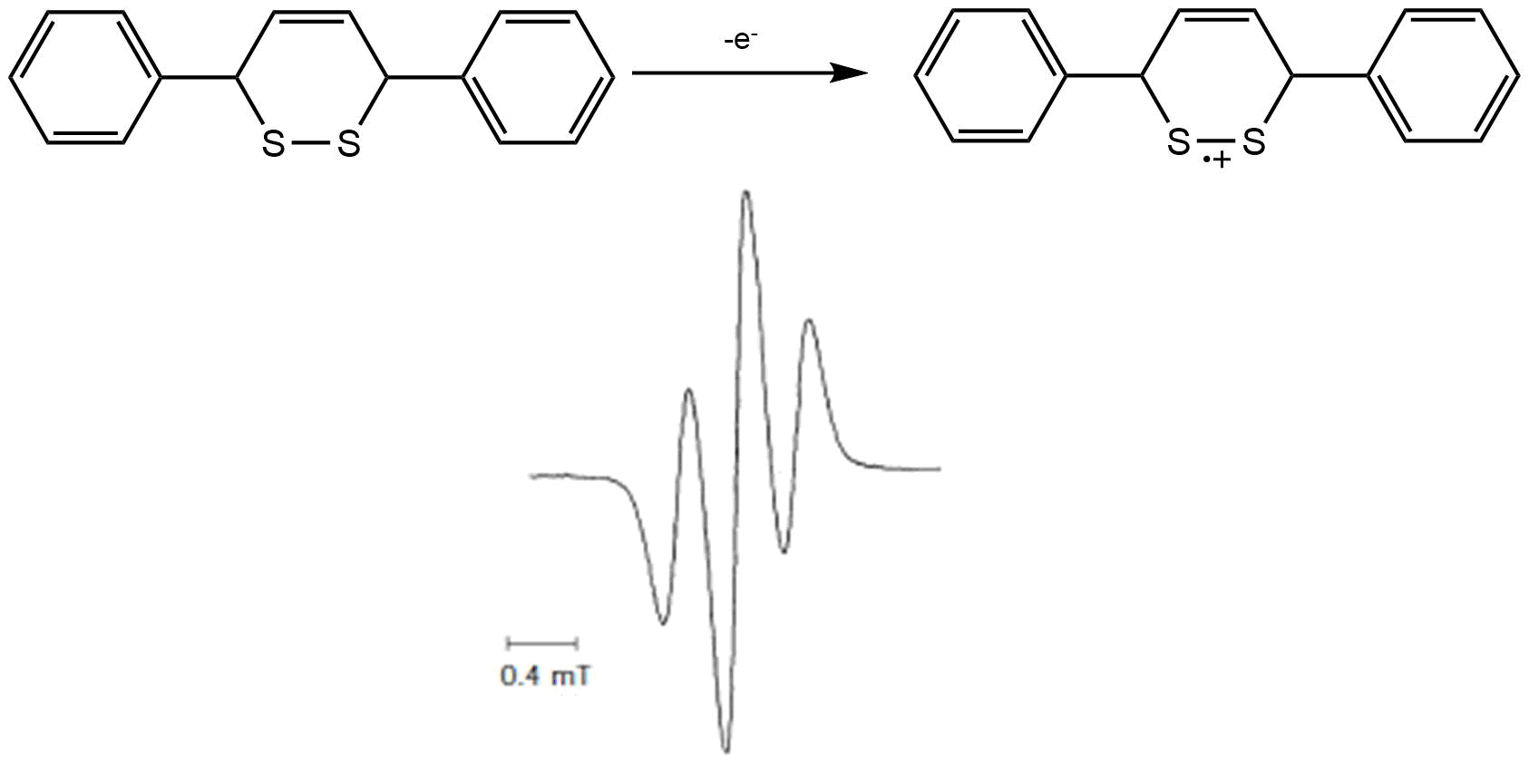
Case Study 1: Thermally-unstable radical cation of 3,6-diphenyl-1,2-dithiine
Electrochemical studies revealed that at E1/2 = + 0.665 V vs Fc/Fc+ the compound undergoes a 1e oxidation to the radical cation. While stable on the short time-scale of CV it was later revealed, by UV-VIS spectroelectrochemistry, that on a time-scale of minutes, the cation decomposes thermally. In order to measure an EPR spectrum of the unstable radical cation it must be cooled to 233 K in DCM. The cell performs well under these conditions, as evidenced by the large signal-to-noise ratio and the rapid apperance of the EPR signal at the potential of the anodic wave. The spectrum indicates that the unpaired electron is largely localized on the sulfur atoms.
Obtain a quote
Contact us to discuss your requirements and obtain a quote for one of our EPR OTTLE cells.
