Room Temperature OTTLE cell
The first version of the Room Temperature OTTLE cell was developed and published in early 1990s (J. Electroanal. Chem. Interfacial. Electrochem. 1991, 317, 3887). The present design features a number of improvements. Several customisable options are available, including modification for 2D IR measurements or ECL. Currently, the cell has been employed in more than 170 research laboratories worldwide and cited in about 800 publications, including the recently issued book Spectroelectrochemistry (RSC, UK, 2009, A. Klein and W. Kaim Eds).
Obtain a quote
Room Temperature OTTLE cell features
A Compact, Modular Design
- Quick to assemble & easy to clean and maintain.
- Wide choice of window materials; CaF2, BaF2, quartz, NaCl, KBr, ZnS, ZnSe, HDPE- diverse (organic, aqueous, acidic aqueous) electrolytes may be used.
- Spectral Range: 50,000 – 50 cm-1
- No leaking and completely air-tight: Ideally suited for studying air- and moisture-sensitive compounds and volatile solvents.

- Low sample volumes required (0.1-0.2 mL).
- Compact design allows for a variety of different holders to be used; ready to use in all types of commercially available UV-Vis-NIR-IR spectrophotometers.
- Wide choice of working electrode materials: Pt, Au, Cu minigrids (32 wires per cm) & graphite sheet – transparent from 2800-1400 cm-1 (coming soon).
- Adaptable for wide range of techniques: resonance Raman (microscope), epifluorescence microscope, ECL technique (using a dual working electrode operated by a bipotentiostat), ECD and VCD spectrometers, fiber optics (UV-vis and IR), 2D-IR laser setup, far-IR region (Bruker Vertex 70v and 80v FT-IR spectrometers).
Outstanding Electrochemical Behaviour

- Rapid electrolysis: suitable for time-resolved measurements and rapid FTIR/UV-Vis spectroscopy scan.
- Negligible diffusion in the thin layer solution (optical path less than 0.2 mm).
- High electrochemical stability with reproducible behavior over several scans.
- Complete conversion of the analyte in the vicinity of the working electrode.
- Low level of electronic noise.
- Cell can be modified to accept input from almost any commercially available potentiostat, our personal recommendation is the PalmSens EmStat3+ range.
Wide-Ranging Application
This cell has been employed with great success on a variety of topics:
Redox Intermediates
Wolfgang Kaim (University of Stuttgart, Germany) and Jan Fiedler (J. Heyrovsky Institute of Physical Chemistry, Prague, Czech Republic) have been the most frequent users of our cells. A large number of their studies deal with identification and characterisation of unusual redox intermediates of mononuclear and mixed-valence dinuclear transition metal complexes. For example, the bridged diruthenium complex below features two o-semiquinone (one-electron-reduced dioxolene) ligands. Combined with theoretical calculations and electron paramagnetic resonance measurements, UV-Vis-NIR spectroelectrochemistry documents dioxolene-based redox activity and variable intra- and intermolecular spin-spin interactions. The predominant Ru(II) oxidation results in diminished intensity and a hypsochromic shift of the NIR band, suggesting largely metal-based spin and mixed MLCT/LMCT charge transfer transitions.
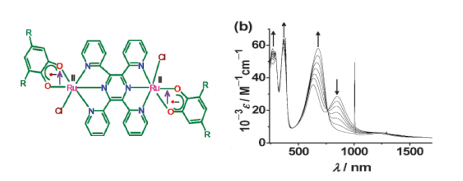
Electronic Properties of Mixed-Valence Complexes
Heinrich Lang and co-workers (Technische Universität Chemnitz, Germany) utilize UV-Vis-NIR spectroelectrochemistry within the RT OTTLE cells to analyze the electron-transfer properties of redox-active thiophene- and fulvalenediyl-bridged homo- and heterodi- and multi-metallic complexes. In particular, below is the structure of supercrowded 2,3,4,5-tetraferrocenylthiophene and several UV-Vis-NIR absorption bands appearing between 280 and 3000 nm when the ferrocenyl terminals in the complex are oxidized in a slow and stepwise fashion. This observation reveals that the positive charges are localized on the Fc+ terminals in the mixed-valent partially oxidized intermediates.

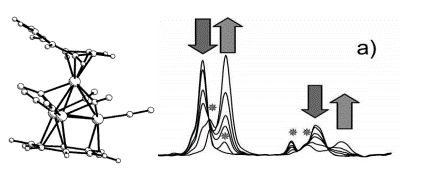
Electron Transfer in Transition Metal Clusters
Carlo Nervi (Dipartimento di Chimica IFM, Università di Torino, Italy) reports the use of IR spectroelectrochemistry in combination with theoretical calculations in a study of the electronic structures and redox properties of dodecahedral transition metal clusters featuring the cyclooctatetraene ligand. Below are the spectral changes of [Co4(CO)6(C8H8)2] in the carbonyl region during the one-electron reduction, which leads to partial decomposition of the bis(cyclooctatetraene) system, as marked with stars.